4th ANNUAL COMPUTER SOFTWARE ASSURANCE 2024
4th ANNUAL COMPUTER SOFTWARE ASSURANCE 2024
About the Event
Having organised two successful virtual editions, Eminence Business Media has thrived in addressing the most critical and crucial challenge areas of the Pharma Industry. Obeying the request of the pharma fraternity and living by the motto, “Learning is a constant process”, to address the building apprehension and calm the nerves of the pharma industry, we are back with an exciting in-person edition.
Eminence Business Media proudly announces the 4th Annual Computer Software Assurance 2024, Live and In-stereo.
Computer System Validation (CSV) was adopted as a new technology by the pharma industry when technological advancements deterred the quality of systems. It has significantly ensured software quality and demonstrated that computer systems work as intended during inspections and audits. On the other hand, Computer Software Assurance (CSA) is said to be a futuristic
mindset based on risk-based critical thinking. The logic promotes simple computerised onboarding with a unique operational context.
The 4th Annual Computer Software Assurance 2024 aims to strengthen and empower these systems. The idea is to create a program that explores the various elemental components to strengthen the CSV gameplan, address the challenge areas to boost the existing system and look at the futuristic mindset of CSA by analysing the knowledge gaps and bursting the myths.
From meeting experts to learning from the regulators them selves, the program is designed to cover different aspects, addressing new challenges, presenting solutions & learning tools that deliver theoretical hypotheses and encourage practical application-based activities.





WHY ATTEND?
Who Should Attend
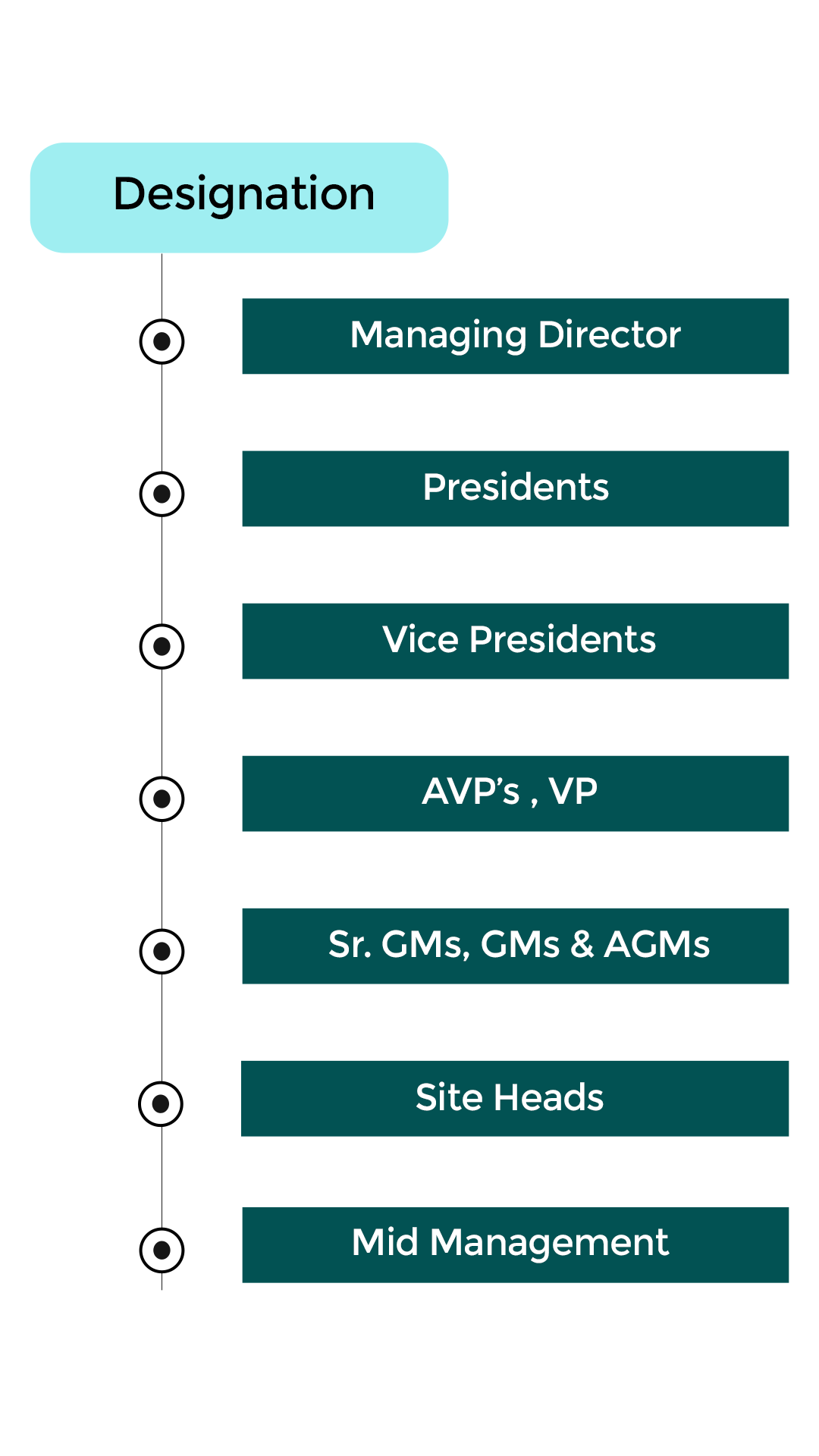

ALSO ANY OTHER PHARMA TEAMS THAT HANDLE COMPUTER SOFTWARE ASSURANCE.
Pricing
- + 18% GST applicable
- + 18% GST applicable
This Pricing is applicable for Pharmaceutical Manufacturing Companies only.*
Past Speakers

Mr. Francisco Vicenty
Program Manager, Case For Quality
FDA

Mr. Khaled Moussally
EVP Clients & Regulatory Relations
Compliance Group

Dr. Mayur Parmar
Deputy Collector, Prant Officer & SDM
Government Of Gujarat

Dr. Vivek Bansal
AVP – QA
Biological E

Mr. Ken Shitamoto
Executive Director, IT Quality and Compliance
Gilead Sciences

Mr. Rajesh T
Head – Digital Transformation – Quality
Dr. Reddy’s

Mr. Sanjeev Dharwadkar
CEO
Pharma Tech Consulting LLP

Dr. Ratnakar Palakodeti
Vice President- Healthcare & Life Sciences
Persistent Systems
Join Us?
Within the current role, you can apply some of the concepts and techniques that will be discussed over two days at the
4th ANNUAL COMPUTER SOFTWARE ASSURANCE 2024
Past Event Partners
Silver Partner

Persistent
With over 22,750 employees located in 21 countries, Persistent Systems (BSE & NSE: PERSISTENT) is a global services and solutions company delivering Digital Engineering and Enterprise Modernization. We work with the industry leaders including 14 of the 30 most innovative companies as identified by BCG, 8 of the top 10 largest banks in the US and India, and numerous innovators across the healthcare and software ecosystems. As a participant of the United Nations Global Compact, Persistent is committed to aligning strategies and operations with universal principles on human rights, labor, environment, and anti-corruption, as well as take actions that advance societal goals.
Exhibit Partner

RxCloud
RxCloud is a professional services organization focused exclusively on Life Sciences and Biotech Industry. We are a specialized consulting and system integrator to assist clients in assessing and implementing IT systems across Quality, Regulatory and Drug Safety space. Our specialization includes reusable frameworks and assets that we have created around integration services, testing and validation services across various suites of products used in Life Science Industry. RxCloud mission is to deliver innovation, customer experience and speed that enables global Health Science industry to continuously innovate and significantly reduce drug development life cycle.
Our Implementation and CSV consultants are immersed experience in performing Implementation, Configuration, Testing, Migration, Upgrades and Validation activities as per the regulations and guidelines set forth by the FDA, EMA and other regulatory bodies.
RxCloud validation consultants are well experienced/knowledge in performing risk-based Function Risk Assessment to determine the risk associated to each functional, process and operational requirements and define the scope of testing and validation required for each requirement in conjunction with draft CSA guideline set forth by FDA. Additionally, Our consultants leverage vendor validation packages and RxCloud automation solutions (RxCloader, RxSmarTest and RxVbot) to reduce validation effort and timeline.

Techsol Life Sciences
Techsol Life Sciences is an integrated clinical development, medical affairs and post-marketing surveillance business solutions provider to global biopharmaceutical, medical device, food, and nutraceuticals companies. With our commitment to bringing novel treatments and therapies faster to market, we deliver regulatory compliant clinical research services, GxP technology consulting and validation services in combination with our unified SaaS platforms. Using our deep-domain scientific expertise and technology innovation, we help sponsors to reduce time-to-market, save costs, and realize maximum value, across pharmaceutical and medical device business functions through insights-driven clinical research, compliance oversight, digitalization and GxP process automation.
Networking Partners

Life Science Consulting Pvt Ltd. (A CONVALgroup COMPANY)
CONVALgroup is a pureplay validation company with over 23 years of record of service. We have offices in Canada, Turkey, Europe (London, Holland, Bratislava) and Pune, India. It’s worldwide staff of over 100+ people have the background and experience in Qualification and validation of Green Field facilities and in project Management ( MES, HVAC, LIMS, ERP, QMS, IT-QA, Track & Trace , BMS etc.)
Life Science Consulting Pvt. Ltd. (LSCPL) is the Indian Entity of CONVALgroup with presence in India since 2012 and has core qualified resources supporting our clients. Additionally, LSCPL has 50+ senior FTEs and workstream leads who are readily available to support clients in India and abroad.
We also have strategic partnerships with Indian and overseas technology and consulting companies like Optel group, Valgenesis, Master Control, Utrace Technologies etc.
CONVALgroup’s specialized services include the use of state-of-the-art cloud based VLMS systems to deliver validated systems by blending its experience in project management and co-ordination with clients. CONVAL’s management techniques include scope creep control, developing agile schedules and using agile validation methodology to meet changing project schedules etc. Using such creative techniques has made CONVALgroup, the “partner of choice” for validation services ( CSV and CSA) for large Life Science companies worldwide such as Novartis, Sandoz, Sanofi, GSK etc.
Our successful track record in delivering, more than 1500 compliance projects in all categories of technical and IT operations, explains our core skillsets and diversity of our expertise.
We at LSCPL also provide end to end Project Management and Validation services for Track & Trace ( Serialization and Aggregation). With our esteemed partners in this domain, we provide these services for CIS countries along with other geographies.

Rephine: The Gold Standard in Life Sciences Product & Device Quality
Rephine are deeply experienced GxP consultants, auditors and practitioners. We proactively help pharmaceutical and medical device companies around the world with all aspects of their manufacturing and supply chain quality assurance and associated business process optimisation.
We have been providing these specialist quality assurance services internationally for more than 25 years, with offices in UK, Spain, India and China.
Our team of highly experienced, top-level consultants carry out key projects for companies around the world in the areas of:
- GxP readiness. Assessing and improving quality or compliance across all phases of the product development and manufacturing lifecycle.
- Digital transformation. Assisting on how to redesign and optimise manufacturing quality processes including Computer Systems Validation and Data Integrity.
- Medical Devices.
- Quality management System (QMS). Advisory service helping our customers to achieve a systematic approach to Quality design and maintenance, and optimised business processes.
Media Partners
Client Reviews
Glimpses
After your feedback at last year’s virtual conference, we were advised to come up with this physical conference on Computer Software Assurance. Few of the glimpses from the last year!!
Send a Message
Partnership Enquiries
Delegate Registration
4th ANNUAL COMPUTER SOFTWARE ASSURANCE 2024
Follow Us